Emission Spectrum of Hydrogen Discrete Not Continuous
Lecture 6: Discrete Spectra of Atoms
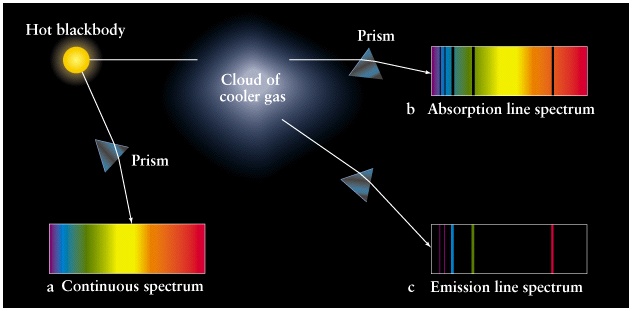
Three types of Spectra
Next
Kirchhoff's Laws
|  |
Back Next
Atomic Spectra: Fingerprints of Atoms
- Each element has a unique set of energy levels where electrons can orbit.
- Each element has a unique set of coloured spectral lines which can be used to identify the element.
- Not all of an elements spectral lines will correspond to EM waves in the visible part of the spectrum.
- for example
- Absorption and emission spectral lines are at exactly the same wavelengths. What you see is determined by environment.
Astronomy: A Hydrogen Emission Nebula
|  |
Astronomy: The Sun's Absorption Spectrum

Some fun links to research-quality Solar spectra:
Interactive Solar Spectrum
Complete Solar Spectrum from 380 to 870 nm
Back Next
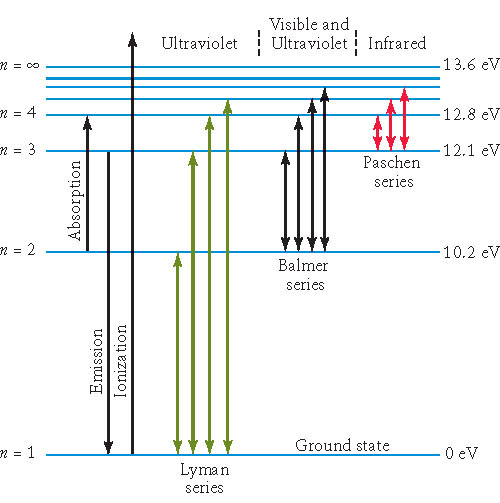
Hydrogen's Spectrum
|  |
Let us practice some computations
We need the following constants| c=3.00 x 108 m | h=6.63 x 10-34 J.s | kB=1.38 x 10-23 J/K | R = 1.10 x 107 m-1 | 1 eV = 1.60 x 10-19 J |
and formulas
Ly-alpha line with longest wavelength ?
Minimum energy to excite hydrogen atom ?
Which jump between close levels is in visible range ?
Back NextHydrogen's Spectrum Balmer lines
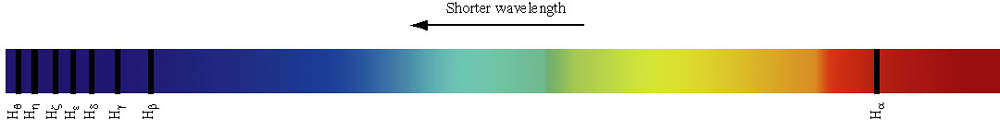
| Name | Initial State | Final State | Colour | Wavelength |
| Balmer alpha | 3 | 2 | red | 656 nm |
| Balmer beta | 4 | 2 | green-blue | 486 nm |
| Balmer gamma | 5 | 2 | blue | 434 nm |
| Balmer delta | 6 | 2 | violet | 410 nm |
Interactive Solar Spectrum: can you find Balmer lines ?
Back Next
Doppler Shift
Waves in water, air ...
| 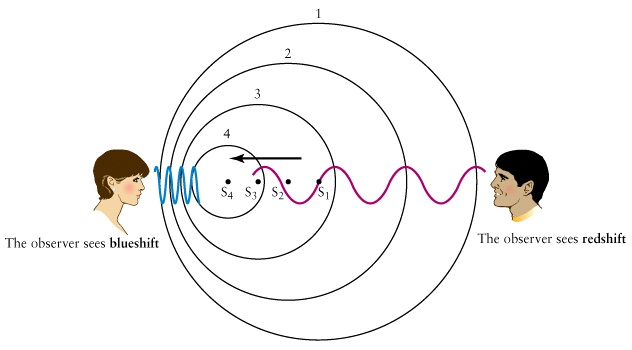 Useful animations 1, 2 Useful animations 1, 2 |
- The change in the wavelength is related to the relative velocity v between the source and the observer and is called Doppler shift given by the formula

- In this equation c is the speed of the wave in the medium.
Electromagnetic waves (light):
- The Doppler shift holds for all types of waves including:
- sound waves (eg. ambulance). Here "c" is speed of sound
- light waves. Here "c" is speed of light.
- Formula for Doppler shift is the same for light, but physics is different - special relativity.
Doppler effect is extremely useful:
- If you can measure the shift in wavelength of a star's spectrum relative to lab you can determine the star's velocity relative to you!
- If you can measure the width of the spectral line in stellar spectrum caused by thermal motions of atoms, you can measure temperature of the star !
Back
Next lecture: Optics and Telescopes
Read Chapter 6
Source: https://sites.ualberta.ca/~pogosyan/teaching/ASTRO_122/lect6/lecture6.html
Postar um comentário for "Emission Spectrum of Hydrogen Discrete Not Continuous"